Animal Physiology
My lab is working in sensory transduction and circadian rhythm research with mainly two model insects, the hawkmoth Manduca sexta and the Madeira cockroach Rhyparobia maderae. I want to understand how insects communicate via odorants, called pheromones and how these pheromones are detected by olfactory receptor neurons (ORNs) on the insect´s antennae. In addition, since ORNs are circadian pacemakers, I want to know how odor detection is modulated daytime-dependently during the sleep wake cycle. My lab is working on olfactory transduction of sex-pheromones of the hawkmoth Manduca sexta and the Madeira cockroach Rhyparobia maderae. During the last four years we succeeded in showing that circadian changes in the concentration of the stress hormone octopamine (the insects norepinephrine) drives circadian rhythms in the antenna´s sensitivity to pheromones (Schendzielorz et al., 2015). Furthermore, we succeeded in analyzing the pheromone transduction cascade (review: Stengl 2017). Male hawkmoths locate their mates via detection of sex-pheromones that the female hawkmoths release in pulsatile fashion. The frequency of pheromone pulses encodes distance to females in upwind direction. The males detect the sex-pheromones with highly sensitive ORNs that innervate long hairs on the insect´s antenna. On the cilia of the ORNs are odor receptors (ORs) that recognize the pheromone ligands, starting a pheromone transduction cascade that finally leads to influx of positive ions into the sensory neuron. Insects, as mammals, employ 7-transmembrane receptor molecules for odor detection. However, the two receptor families are not related since the insect receptors, compared to the mammalian receptors, are inversely inserted into the plasma membrane. The co-occur with a second protein, called Orco (olfactory receptor co-receptor). Orco forms cation channels if expressed in heterologous expression systems. The functions of Orco in pheromone transduction is under debate. While everybody agrees that Orco locates and maintains ORs in the membrane of the cilia, it is under debate whether Orco participates as a ligand-gated receptor-ion channel complex directly in the odor transduction cascade. Mostly based upon heterologous expression studies it was followed that odor transduction involved first the ligand-gated opening of OR-Orco. Others found an additional G-protein dependent cascade initiated via OR-odor interactions. In the hawkmoth, however, we found no evidence for Orco in an ionotropic pheromone transduction cascade, but found G-protein coupled metabotropic pheromone transduction. Thus, we suggested a new hypothesis of Orco functions as second messenger- and voltage-gated pacemaker channel (Stengl 2017; Nolte et al., 2016). In addition, together with collaborators at the MPI in Jena we identified the pheromone receptor in the hawkmoth (Wicher et al., 2017) and characterized pheromone transduction channels pharmacologically (Gawalek et al., 2018).
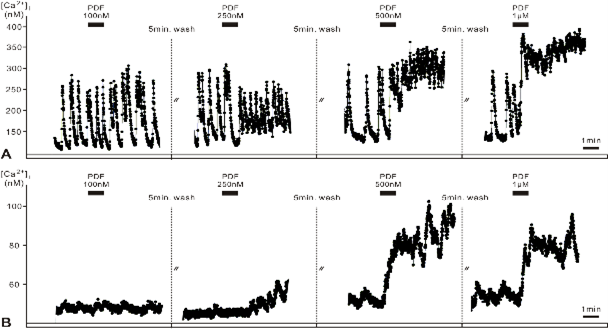
In my second field of study, my lab analyzes the circadian clock of the Madeira cockroach (reviews: Stengl et al., 2015; Stengl and Arendt, 2016). In a CINSaT collaboration with the lab of Dr. Cyril Popov we could adhere circadian pacemaker neurons of the Madeira cockroach on ultra-nano-crystalline diamond surfaces much stronger that with other procedures employed before. This made it possible for the first time to not only physiologically characterize primary cell cultures of circadian pacemaker neurons but also characterize them in immunocytochemical experiments (Voss et al., 2016; Gestrich et al., 2018). Thus, for the first time we could demonstrate that circadian pacemaker neurons of the insect clock express autoreceptors for their own neuropeptide pigment-dispersing factor (PDF) which is the most important circadian coupling signal in the insect circadian clock (Gestrich et al., 2018). These data allowed us to develop a new hypothesis of circadian clock function in the control of sleep-wake cycles. Furthermore, with multiple-label immunocytochemistry we further characterized the cellular network of the insect circadian clock (Arendt et al., 2016, 2017; Giese et al., 2018a, b). Finally, in CINSaT collaborations with Dr. Garcia´s lab and the lab of Dr. Herzel at the Charite in Berlin we started an in depth analysis of the circadian clock network and how it controls sleep wake cycles in the Madeira cockroach (Rojas et al., submitted). In summary, we considerably progressed in both of our scientific projects, also made possible with collaborations within CINSaT.
Prof. Dr. Monika Stengl
full member

- Telephone
- +49 561 804-4564
- stengl[at]uni-kassel[dot]de
- Location
- Universität Kassel
Fachbereich 10 - Naturwissenschaften & Mathematik
Institut für Biologie
Heinrich-Plett-Straße 40
34132 Kassel
- Room
- 2286
