Organometallic Chemistry
A. R. Petrov, A. Derheim, J. Oetzel, M. Leibold, C. Bruhn, S. Scheerer, S. Oßwald, R. F. Winter, U. Siemeling, Inorg. Chem.2015, 54, 6657.
“A Stable Planar-chiral N-Heterocyclic Carbene with a 1,1´-Ferrocenediyl Backbone”
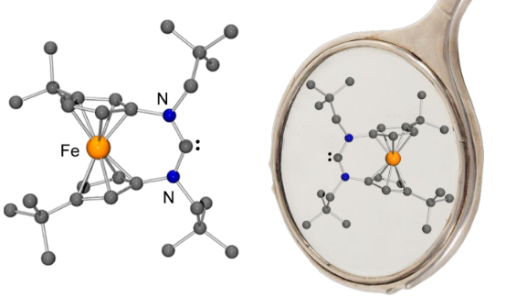
A stable N-heterocyclic carbene with a redox-active 3,3’-di-tert-butylferrocenylene backbone was synthesized and investigated. The tert-butyl groups significantly enhance the solubility of the carbene and its precursor compounds in aprotic organic solvents. In addition, they cause the planar chirality of this singlet carbene, whose pronounced ambiphilicity makes it suitable for the activation of fundamentally important small molecules like CO.
F. Ahrend, U. Glebe, L. Árnadóttir, J. E. Baio, D. A. Fischer, C. Jaye, B. O. Leung, A. P. Hitchcock, T. Weidner, U. Siemeling, A. Ehresmann, Langmuir2016, 32, 10491.
“Magnetic field landscapes guiding the chemisorption of diamagnetic molecules”
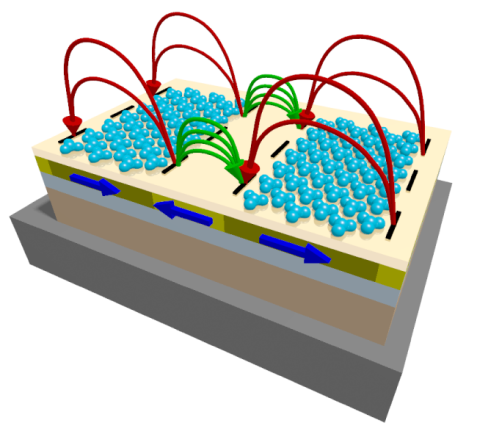
The self-assembly of diamagnetic molecule sub-monolayers on a surface can be influenced by magnetic stray field landscapes emerging from artificially fabricated magnetic domains and domain walls. The directed local chemisorption of diamagnetic molecules in relation to the artificially created domain pattern is proved by a combination of surface analytical methods: ToF-SIMS, X-PEEM and NEXAFS imaging.
M. Liebscher, C. Bruhn, U. Siemeling, J. Baio, H. Lu, T. Weidner, Eur. J. Inorg. Chem.2017, 351.
“The Interaction of 1,1’-Diphosphaferrocenes with Gold: Molecular Coordination Chemistry and Adsorption on Solid Substrates”
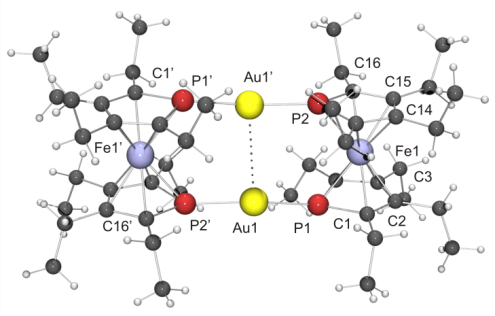
The binding behaviour of 1,1’-diphosphaferrocenes towards gold has been studied in molecular and surface coordination chemistry with pristine 1,1’-diphosphaferrocene and five congeners bearing up to eight alkyl or aryl substituents. Self-assembled monolayers and molecular AuI complexes exhibiting unprecedented intermetallic interactions are described.
L. Wallbaum, D. Weismann, D. Löber, C. Bruhn, P. Prochnow, J. E. Bandow, U. Siemeling, Chem. Eur. J.2019, 25, 1488.
“Stable and Persistent Acyclic Diaminocarbenes with Cycloalkyl Substituents and Their Transformation to b-Lactams by Uncatalysed Carbonylation with CO”
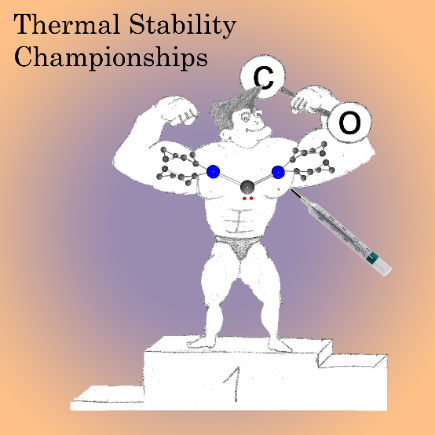
Acyclic diaminocarbenes (ADACs) are ambiphilic, which makes them suitable for the activation of fundamentally important small molecules. For example, most of them react with CO under mild conditions. Unfortunately, such high reactivity is accompanied by limited thermal stability, leading to their decomposition at room temperature or below. The new ADAC [(cyclo-C6H11)2N]2C is stable in solution up to ca. 40 °C. Nevertheless, it reacts readily with CO. The carbonylation affords a b-lactam derivative with useful antibiotic properties.
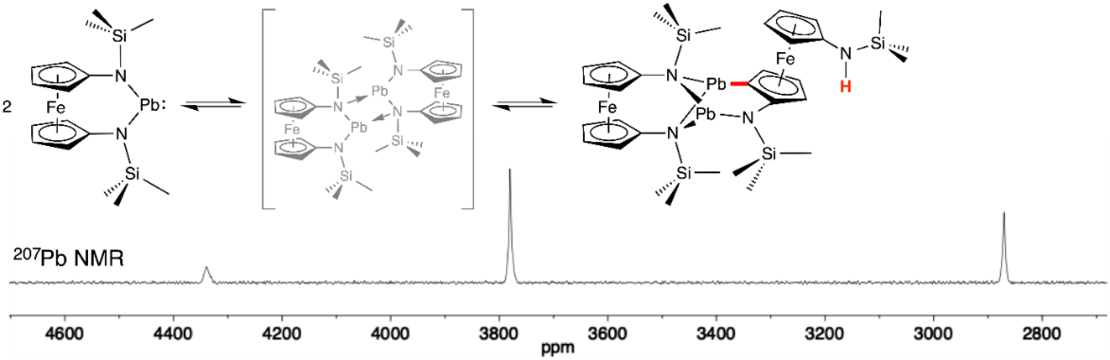
R. Guthardt, J. Oetzel, J. I. Schweizer, C. Bruhn, R. Langer, M. Maurer, J. Vícha, P. Shestakova, M. C. Holthausen, U. Siemeling, Angew. Chem.2019, 131, 1401; Angew. Chem. Int. Ed.2019, 58, 1387.
“Reactive Dimerization of an N-heterocyclic Plumbylene: C-H Activation with PbII”
An unprecedented type of dimerization is shown by an N-heterocyclic plumbylene with ferrocenylene backbone. The initially formed aggregational dimer undergoes an intramolecular electrophilic substitution by an endo attack of a PbII atom, causing the cleavage of a C-H bond and the formation of a Pb-C and an N-H bond. The reaction affords a planar-chiral ferrocene derivative and is the first example of a C-H activation with PbII.
Prof. Dr. Ulrich Siemeling
full member

- Telephone
- +49 561 804-4576
- siemeling[at]uni-kassel[dot]de
- Location
- Universität Kassel
Fachbereich 10 - Naturwissenschaften & Mathematik
Institut für Chemie
Heinrich-Plett-Straße 40
34132 Kassel
- Room
- 2251
