WP3-4 Chemical Aspects of New Storage Materials
Numerical investigation on closed adsorption systems
| Hosted by: | University of Kassel |
|---|---|
| Student: | Kristijan Krekic |
| Started: | 01/02/2014 |
| Supervisor(s): | Prof. Dr. Rudolf Pietsching |
Short description
Several materials were investigated by isothermal titration calorimetry. Prior to the titration experiment desorption of water was carried out by heating and evacuation in a sealed tube. The applied temperatures range from 100°C (for silica gel) to 200 °C (for zeolite 13XBF) in order to fully dry the specific material. According to the titration experiments zeolite 13XBF shows the best adsorption enthalpy (integration of whole adsorption range). MOF-199, even though it has a lower adsorption enthalpy desorbs water at a much lower temperature of 120 °C, rendering it better suited for regeneration processes. Calcium chloride modified silica materials (SBA-15) show increasing adsorption with larger contents of CaCl2. MOFs with structural similarities to zeolites, MIL-101-M3 (M= Fe, Cr, Al), show a lower adsorption then zeolite, albeit lower desorption temperature (140 °C) and higher water uptake. Also aluminum phosphate, APO-TRIC-12, shows large adsorption enthalpy with low desorption temperature.
Project background (click to open)
We are especially interested in (ad-)sorption processes involving solid or liquid phases which can be carried out in open or closed systems. Some of these systems are commercially available to date, however the sorption materials currently in use are subject of improvement. In this Ph.D. project in the Chemical Hybrid Materials group in Kassel the focus is set to the development and modification of novel desiccant sorption materials which may be solid, liquid or a combination of both. Existing solid sorption phases suffer from limited sorption capacities, mediocre thermal properties and space-filling as well as diffusion limited mass transport from the surface to the bulk material. On the other hand liquid desiccant phases are often corrosive, toxic and expensive. Therefore, in addition to established storage materials the potential of new types of solid and liquid desiccants like metal organic frameworks (MOFs) and ionic liquids (ILs) are explored aiming at applications involving energy storage and conversion. In the course of the project materials and material combinations are assessed based on literature data (if available) but also experimentally (e.g. isothermal titration calorimetry, water vapor pressure, desorption conditions, etc.). To improve their desirable properties, structural modifications on a molecular level will be carried out to optimize physical properties relevant to heat and mass transfer as well as to storage capacity.
Methodology (click to open)
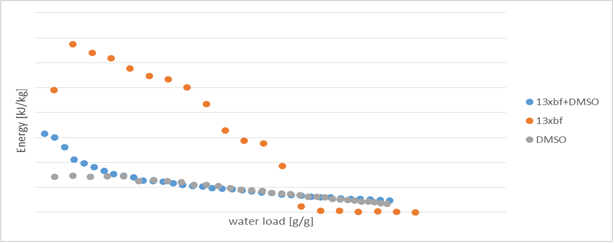
Current experiments are based on improving poor heat conduction of solid materials. For that we are introducing a new model. By using combination of solid and liquid we are not only improving heat conduction of system but also the water load capacity. Until now several liquids were tested in combination with zeolite 13XBF. First test show an increase in heat conduction and water capacity but water load on zeolite is reduced due to competition of liquid and water. Based on this results dimethyl sulfoxide (DMSO) as liquid was used, showed on fig 1. Future experiments will be carried using different liquids in order to find best potential match.
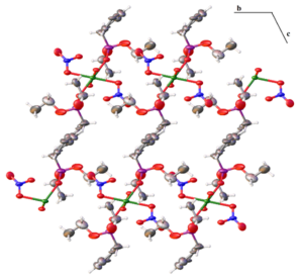
Parallel to adsorption investigation experiments to prepare new porous materials are made. For this purpose Phosphonic acid bridging ligands were selected and are complexed with lanthanide metals. First results show formation of coordination polymers (COPs) in various polymer types depending on properties of metal selected. Example structure showed on Fig 2.
