Biochemistry
Research on intracellular signal transduction and biosensing.
Protein kinases transfer phosphate groups to target molecules (see 4.) and are key enzymes in signal transduction in cells involved in major human diseases like cancer (Espiard et al JCI Insight 2018), neurodegenerative diseases like Parkinson’s disease (Schmidt et al PNAS 2019, see 3.) as well as in human pathogens like the Malaria parasite Plasmodium (Franz et al 2018: see 1.)
Investigating conformational changes governing protein dynamics in kinases (immobilization strategies with Ehresmann; protein dynamics calculations and Markov State Modelling with Garcia)
Protein kinases undergo distinct conformational states and conformational control has been established as a concept for kinase regulation. We are investigating the underlying molecular mechanisms at the atomic level employing biophysical techniques as well as biochemical assays, supported by protein dynamics calculations. Chemical modifications of magnetic nanoparticles (mnps) and of biomolecules are used to produce mnp-protein conjugates to study binding events as well as to quantify conformational changes. This has successfully been applied to nanocrystalline diamonds- protein conjugates (Merker et al 2018). Emerging technology i.e. switchSENSE technology is being established to analyze conformational changes on different scales.
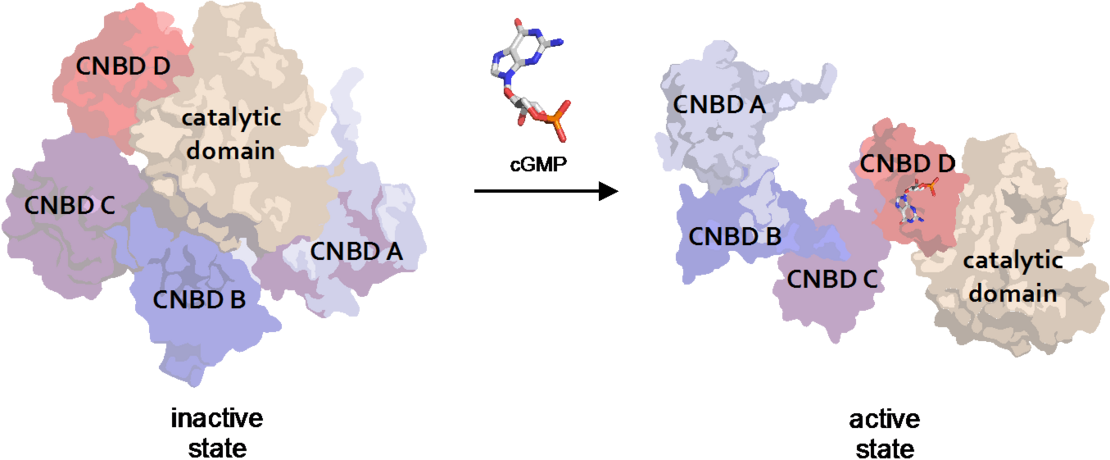
Figure 1. cGMP dependent Protein Kinase from the malaria-causing parasite Plasmodium falciparum (PfPKG, PDB 5DYK) undergoes rather large conformational changes (nm-range) upon binding of the second messenger cGMP (Franz et al 2018)
References:
Merker D et al. Nanostructured modified ultrananocrystalline diamond surfaces as immobilization support for lipases Diamond & Related Materials 90 (2018) 32–39
Franz E, Knape MJ, Herberg FW. cGMP binding domain D mediates a unique activation mechanism in Plasmodium falciparum PKG. ACS Infect Dis. 2018 Mar 9;4(3):415-423.
First description of a protein motif controlling kinase function and stability (in collaboration with U. California San Diego and the National Center for Microscopy and Imaging Research, San Diego)
Leucine-rich repeat kinase 2 (LRRK2) is a large multidomain protein linked to Parkinson’s Disease, however, the regulation of this protein kinase and the underlying pathogenic mechanisms are elusive. Two of the five most common familial LRRK2 mutations (G2019S and I2020T) are localized to the conserved DFGψ (ψ any hydrophobic residue) motif in the kinase core. We discovered a major regulatory mechanism embedded in the kinase domain crucial for the assembly of the regulatory kinase spine (RS) and show that the DFGψ motif serves as a conformational switch that drives LRRK2 activation.
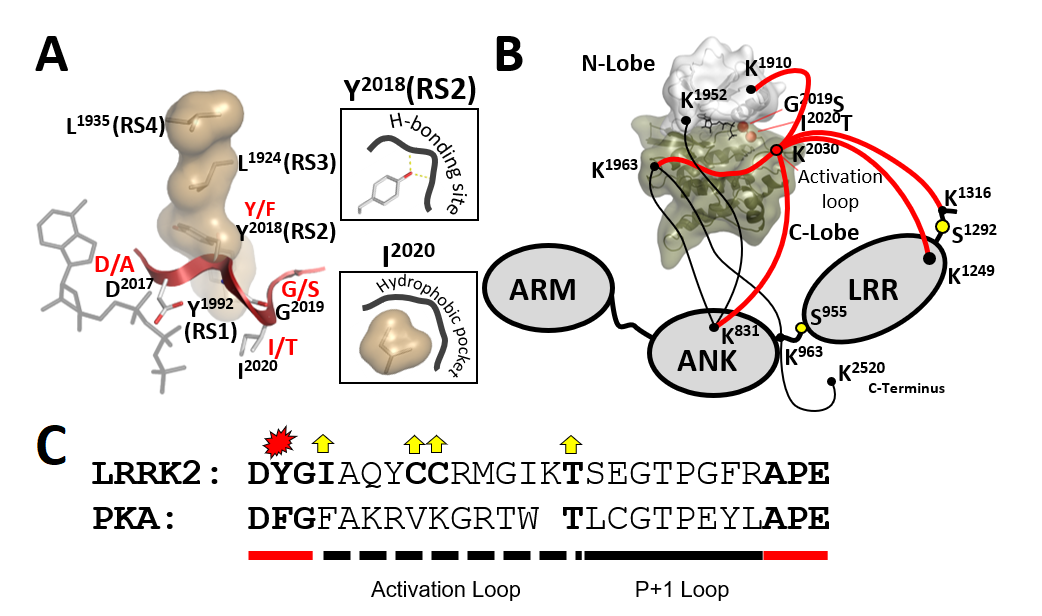
Figure 2. The assembly of the R-spine needs to be tightly regulated to control the conformation of the DYGψ motif and thereby, the ON and OFF states of the kinase. Pathogenic mutations in this motif (i.e., G2019S [DYGψ] and I2020T [DYGψ]) alter kinase regulation and become a driving force for Parkinson´s Disease. With Y2018F (RS2/ DYGψ), we describe a mutation resembling features of G2019S and I2020T.
Reference:
Schmidt SH, Knape MJ, Boassa D, Mumdey N, Kornev AP, Ellisman MH, Taylor, SS and Herberg FW The dynamic switch mechanism that leads to activation of LRRK2 is embedded in the DFGψ motif in the kinase domain Proc Natl Acad Sci U S A 2019 Jul 23;116(30):14979-14988.
Investigation of conformational dynamics in the catalytic subunit (C) of protein kinase A (PKA-C).
PKA-C undergoes conformational changes in the subnanometer range. We used a novel method based on Surface Plasmon Resonance to reveal the role divalent metal ion in conformational control of protein kinases. In particular the Calcium, crucial for muscle contraction, seems to play an essential role protein kinase regulation (Knape et al ACS Chem Biol. 2015, Knape et al Metallomics 2017, Zhang et. al PLoS Biol. 2015).
References:
Knape MJ, Ballez M, Burghardt NC, Zimmermann B, Bertinetti D, Kornev AP and Herberg FW Divalent metal ions control activity and inhibition of protein kinases Metallomics 2017 Nov 15;9(11):1576-1584
Knape, MJ, Ahuja, LG, Bertinetti, D, Burghardt, NCG, Zimmermann, B, Taylor, SS, Herberg, FW Divalent metal ions Mg2+ and Ca2+ have distinct effects on protein kinase A activity and regulation ACS Chem Biol. 2015 Oct 16;10(10):2303-15
Zhang P, Knape MJ, Ahuja LG, Keshwani MM, King CC, Sastri M, *Herberg FW, *Taylor SS. (2015) Single Turnover Autophosphorylation Cycle of the PKA RIIβ Holoenzyme. PLoS Biol 13(7): e100219
New research consortium on mechanisms of kinases and phosphotransfer (PhosMOrg) funded by the program “future” of Kassel University (Schaffrath, Herberg, Müller, Fuhrmann-Lieker, Garcia)
Modification of proteins by addition or removal of phosphates (phosphorylation/dephosphorylation) constitutes an important means for cells to regulate processes. Phosphomodifications are also crucial for synthesis of complex biominerals. Phosphorylation by dedicated enzymes (protein kinases) can alter the charge or conformation of the modified protein, eventually changing its biological activity. Therefore, protein kinases represent molecular ‘on/off’ switches that orchestrate chemical signals with proper cellular behavior (Fig.) PhosMOrg has teamed-up CINSaT members with interdisciplinary expertise in kinase biology (Herberg, Müller, Schaffrath), nanochemistry (Fuhrmann-Lieker) and biomolecular modelling (Garcia).
Prof. Dr. Friedrich W. Herberg
full member

- Telephone
- +49 561 804-4511
- herberg[at]uni-kassel[dot]de
- Location
- Universität Kassel
Fachbereich 10 - Naturwissenschaften & Mathematik
Institut für Biologie
Heinrich-Plett-Straße 40
34132 Kassel
- Room
- 2161
