Zoology
Insights into the “superglue” of velvet worms
The biological “superglue” of velvet worms (Fig. 1) provides inspiration towards circular processing of advanced polymers (Baer et al. 2019a, 2019b). In nature, velvet worms employ a fluid, protein-rich secretion for hunting and defense, which forms rapidly into stiff fibers (Fig. 1). The fluid-to-fiber transition occurs outside the body without regulations, indicating that the “instructions” for assembly are programmed into the protein-building blocks. Electrostatic interactions between oppositely charged protein domains and free ions drive protein folding, self-organization (coacervation) and stabilization of the building blocks into nanoscale droplets (Baer et al. 2019b, 2019c). Yet, nanodroplets can be instantly transformed via simple mechanical stimulus as proteins partially unfold, merge together and form a strong network, which solidifies into a fiber. The mechanism is based on basic physico-chemical principles. Thus, by extracting these principles, new methods of synthesizing sustainable polymer-based materials can be developed.

X-ray imaging of a water bear
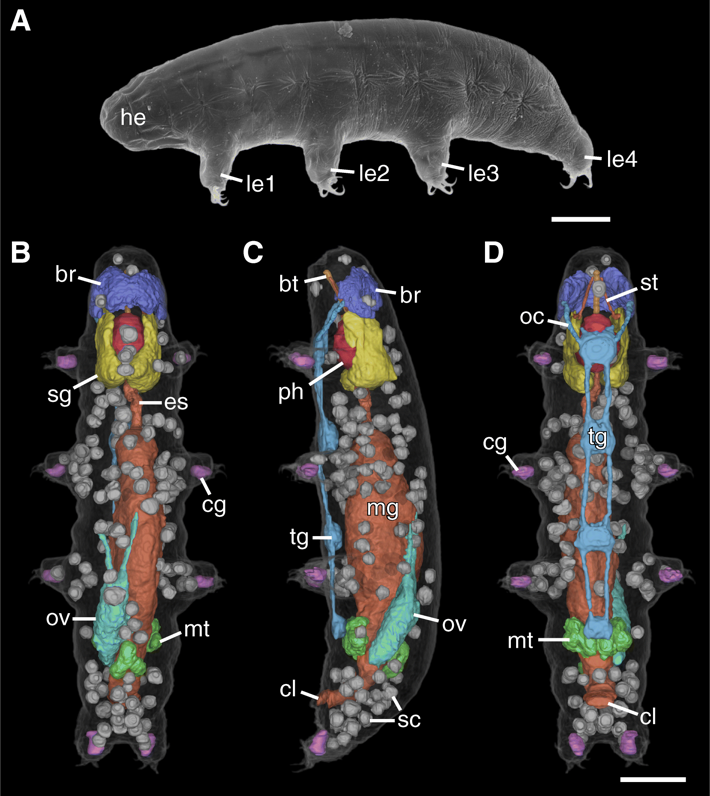
Tardigrades (water bears: Fig. 2A) are miniaturized invertebrates (Gross et al. 2019a) of which the anatomy has been well studied using traditional techniques, but a comprehensive three-dimensional reconstruction has never been performed. In order to close this gap, we employed X-ray computed tomography (CT), a technique that is becoming increasingly popular in zoology for producing high-resolution, three-dimensional (3D) scans of whole specimens. While CT has long been used to scan larger samples, its use in some microscopic animals can be problematic, as they are often too small for conventional CT yet too large for high-resolution, optics-based soft X-ray microscopy. This size gap continues to be narrowed with advancements in technology, with high-resolution imaging now being possible using both large synchrotron devices and, more recently, laboratory-based instruments. We therefore have used a recently developed prototype lab-based nano-computed tomography device to image a 152 μm-long tardigrade at high resolution (200–270 nm pixel size) (Gross et al. 2019b). The resulting dataset allowed us to visualize the anatomy of the tardigrade in 3D and analyze the spatial relationships of the internal structures. Segmentation of the major structures of the body enabled the direct measurement of their respective volumes. Furthermore, we segmented every storage cell individually and quantified their volume distribution. Our data demonstrate the utility of CT imaging as a powerful supplementary tool for studies of tardigrade anatomy, especially for quantitative volume measurements. This nanoCT study represents the smallest complete animal ever imaged using CT, and offers new 3D insights into the spatial relationships of the internal organs of water bears.
References
Baer, A., Schmidt, S., Mayer, G. & Harrington, M.J. (2019a): Fibers on the fly: Multiscale mechanisms of fiber formation in the capture slime of velvet worms. Integrative and Comparative Biology. In press.
Baer, A., Oliveira, I.S., Schmidt, S., Harrington, M.J. & Mayer, G. (2019b): Mechanismen der Natur als Vorbild für nachhaltige Biomaterialien: Einblicke in den „Sekundenkleber“ der Stummelfüßer. Biologie in unserer Zeit. In press.
Baer, A., Horbelt, N., Nijemeisland, M., Garcia, S.J., Fratzl, P., Schmidt, S., Mayer, G. & Harrington, M.J. (2019c): Shear-induced β-crystallite unfolding in condensed phase nanodroplets promotes fiber formation in a biological adhesive. ACS Nano 13:4992–5001.
Gross, V.*, Treffkorn, S.*, Reichelt, J., Epple, L., Lüter, C., & Mayer, G. (2019a): Miniaturization of tardigrades (water bears): Morphological and genomic perspectives. Arthropod Structure and Development 48:12–19. *Equal contribution.
Gross, V.*, Müller, M.*, Hehn, L., Ferstl, S., Allner, S., Dierolf, M., Achterhold, K., Mayer, G.**, & Pfeiffer, F.** (2019b): X-ray imaging of a water bear offers a new look at tardigrade internal anatomy. Zoological Letters 5:14. * and ** equal contributions.
Prof. Dr. Georg Mayer
full member

- Telephone
- +49 561 804-4805
- georg.mayer[at]uni-kassel[dot]de
- Website
- Zoologie
- Location
- Universität Kassel
Fachbereich 10 - Naturwissenschaften & Mathematik
Institut für Biologie, Fachgebiet Zoologie
Heinrich-Plett-Str. 40
34132 Kassel
