Biological Clocks
Due to the outstanding electrochemical properties, superior chemical inertness and biocompatibility, etc., diamond in a form of thin films with different crystallinities has been recognized as an extremely attractive material for (bio-)chemical sensing and as an interface to biological systems. The ultrananocrystalline diamond (UNCD) films deposited in our group by microwave plasma chemical vapor deposition from methane/nitrogen gas mixtures are composed of diamond nanocrystallites of up to 10 nm diameter embedded in an amorphous carbon matrix. Their surface is smooth with rms roughness of 10 – 14 nm and H-terminated, rounded nanostructures with diameters up to 100 nm dominate the topography. After surface modifications by different plasma and photochemical processes the UNCD surface can be rendered strongly hydrophilic (with O- or NH2-termination) or strongly hydrophobic (with F-termination). Furthermore, the surface termination can be patterned.
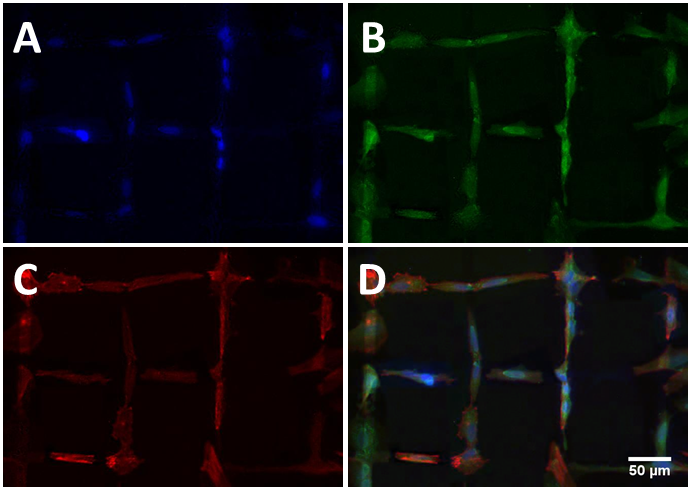
The biocompatibility of UNCD films was studied by direct contact tests with several cell lines. All cells showed good adhesion and spreading on the as-grown UNCD surfaces following the incubation. Comparisons with cells from control samples after several days of cultivation revealed that the UNCD films are not cytotoxic and do not affect the cell viability and proliferation. Further, the influence of the surface termination on the cell attachment and proliferation was investigated. The cells showed enhanced attachment on the modified O- and NH2-terminated UNCD films which could be attributed to an enlargement of the contact area of the cells on the hydrophilic surfaces [1]. The surface roughness of the UNCD is low enough on the scale of the cells to constrain their spreading, enhancing on the other hand the cell adhesion due to the nanostructured surface which is not affected by the modification processes [2]. The patterning of the surface termination leads to guided cell attachment and growth (see the figure) [3].
The UNCD surfaces can be functionalized with proteins or nucleic acids after various chemical modifications depending on the surface termination. For examples, we have demonstrated the attachment of dsRNA on UNCD surfaces functionalized with thiol-terminated DNA, the covalent bonding of esterase and the non-covalent bonding of lipase [4].
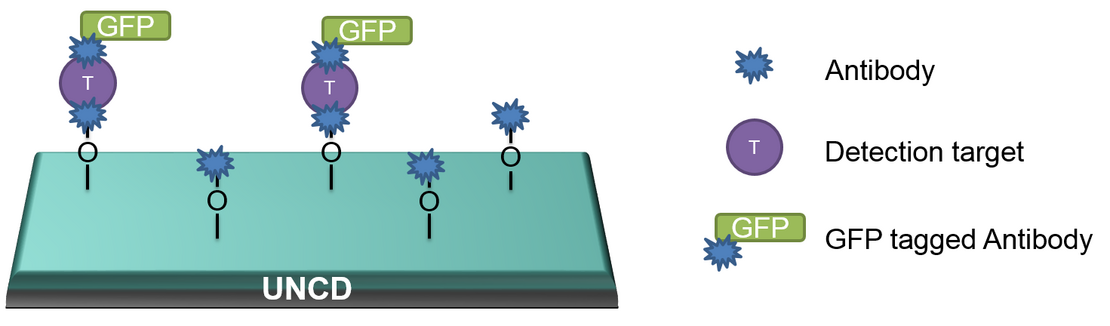
The goal of our project is the preparation of UNCD-based platforms for fluorescence study of time-resolved signals from neurons. The UNCD surface will be modified in a patterned way in order to define the positions for enhanced cell attachment. At the same time the area around these positions will be functionalized with antibodies against neurotransmitters (acetylcholine, , serotonin) or neuropeptides (pigment-dispersing factor) secreted from the cells. These targets will interact with antibodies marked with green fluorescence protein (GFP) and optically detected with a confocal microscope in a given time range (see the figure). As an alternative to such sandwich constructs, a competitive immunoassay (e.g. for serotonin) can be applied. The project will be realized in cooperation with the Department of Animal Physiology and the Department of Biochemistry.
1. A. Voss, H. Wei, C. Müller, C. Popov, W. Kulisch, G. Ceccone, C. Ziegler, M. Stengl, J.P. Reithmaier, Influence of the surface termination of ultrananocrystalline diamond/amorphous carbon composite films on their interaction with neurons, Diamond and Related Materials 26 (2012) 60-65.
2. A. Voss, H. Wie, Y. Zhang, S. Turner, G. Ceccone, J.P. Reithmaier, M. Stengl, C. Popov, Strong attachment of circadian pacemaker neurons on modified ultrananocrystalline diamond surfaces, Materials Science and Engineering C 64 (2016) 278-285.
3. A. Voss, S. Stateva, J.P. Reithmaier, M.D. Apostolova, C. Popov, Patterning of the surface termination of ultrananocrystalline diamond films for guided cell attachment and growth, Surface and Coatings Technology 321 (2017) 229-235.
4. D. Merker, M. Kesper, L.L. Kailing, F. Herberg, J.P, Reithmaier, I.V. Pavlidis, C. Popov, Nanostructured modified ultrananocrystalline diamond surfaces as immobilization support for lipases, Diamond and Related Materials 90 (2018) 32-39.
Project Partners
- Prof. Dr. Monika Stengl, Dept. of Tierphysiologie/Neuroethologie
- Prof. Dr. Arno Müller, Dept. of Entwicklungsgenetik
- Prof. Dr. Olaf Stursberg, Dept. of Regelungs- und Systemtheorie
- Prof. Dr. Raffael Schaffrath, Dept. of Mikrobiologie
- Prof. Dr. Georg Mayer, Dept. of Zoologie
- Prof. Dr. Jörg Kleinschmidt, Dept. of Biophysik
- Prof. Dr. Markus Maniak, Dept. of Zellbiologie
- Prof. Dr. Friedrich Herberg, Dept. of Biochemie
- Prof. Dr. Martin Garcia, Dept. of Theoretische Physik
- Prof. Dr. Thomas Fuhrmann-Lieker, Dept. of Physikalische Chemie
Picture gallery