Project 2
P2: Role of TOR and tRNA mediated translational regulation as coupling factor between multiscale clocks
Collaborators
- PI Prof. Dr. Friedmann (Mathematics) (öffnet neues Fenster)
- PI Prof. Dr. Fuhrmann-Lieker (Chemistry) (öffnet neues Fenster)
- PI Prof. Dr. Gutekunst (Biology) (öffnet neues Fenster)
- PI Dr. Kapp & Prof. Dr. Müller (Biology) (öffnet neues Fenster)
- PI PD Dr. Neupert (Biology) (öffnet neues Fenster)
- PI Prof. Dr. Seiler (Mathematics) (öffnet neues Fenster)
- PI Prof. Dr. Stursberg (Electrical engineering/Informatics) (öffnet neues Fenster)
Project description
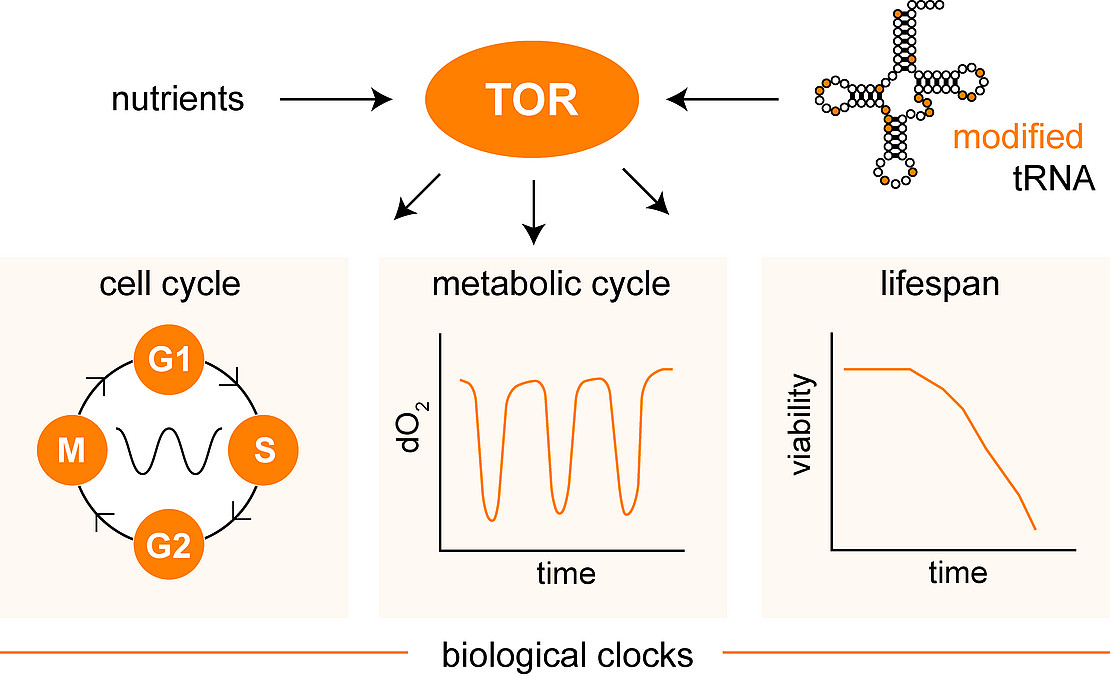
Budding yeast Saccharomyces cerevisiae is a unicellular eukaryote employed in the study of distinct biological clocks at multiple times scales. Well studied examples are the the cell division cycle with a period of ~2 h and rhythmic oscillations in metabolism and oxygen consumption (metabolic cycles) with a period of ~4 h. In addition, the life span of individual budding yeast cells is also controlled by a genetically determined biological clock operating at infradian time scales. Rhythmic gene expression is a key element for the function of biological clocks and involves rhythmic formation and decoding of mRNAs. The decoding process employs abundantly modified transfer RNA (tRNA) and defects in the correct modification patterns of tRNA appears to disrupt the correct timing and synchronization between several biological oscillators, but mechanisms and principles remain obscure. In humans, mutation of tRNA modification genes is associated with severe defects in neurodevelopment and normal cognition, underscoring the relevance of proper tRNA modification for cell function and human health.
Molecular commitment steps define the entry into new, repetitive division cycles which are coordinated with the available nutrient supply in the model system S. cerevisiae. A central evolutionary conserved protein kinase complex termed TOR (target of rapamycin) regulates several distinct cellular responses to nutrient availability. This includes the regulation of translation and also the control of cellular lifespan and cell cycle commitment. Intriguingly, defective tRNA modification in yeast leads to disturbed TOR signaling on the one hand and uncoupling of cellular oscillators on the other, suggesting an ill-defined role of tRNA modification in the synchronization of biological clocks at multiple time scales [Figure 1].
In this project we aim to characterize how tRNA modification links TOR signaling with cell cycle or metabolic oscillators and the control of lifespan and aging. The mechanism how tRNA modification is involved in the coupling between these different oscillators remains to be established and represents a major aim of the project.