Project 8
P8: Second messenger oscillations of cAMP and calcium control biological clocks
Collaborators
Project description
Second messengers time highly accurate cellular responses to external stimuli. First messengers such as the zeitgeber signals or coupling factors like hormones bind to G-protein coupled plasma membrane receptors (GPCRs). This initiates an amplification machinery that translates the external signals into a set of highly diverse intracellular second messengers like cAMP or Ca2+. Second messengers are not only controlled in the time domain. They are also strictly compartmentalized within a cell and not simply distributed by diffusion and may be localized in membrane free cellular sub-compartments. Furthermore, cAMP and Ca2+ oscillate in amplitude and frequency and their oscillations are coupled. Pools of cAMP that are coupled via proteins to the plasma membrane (PM) oscillate out-of-phase with free cAMP pools in the cytosol, while Ca2+ levels oscillate uniformly in-phase throughout the entire cell. cAMP-dependent kinase (PKA), as the primary receptor for cAMP, is a “master controller” for intracellular Ca2+ concentrations i.e. Voltage Gated Ca2+ Channels (VGCA). Disruption of clusters embedding cAMP generating adenylyl cyclases (ACs) and PKA anchoring proteins (AKAPs) disturbs uniform Ca2+ oscillations.
We propose a model of temporal-spatial control of second messengers, their synthesizing or degrading enzymes and kinases governs continuous temporal information processing of the plasma membrane clock with superimposed mechanisms at different time scales (ms-hrs). It allows endogenous cellular rhythms to resonate with multiscale environmental rhythms, controlling neuropeptide release to govern behavioral/physiological rhythms.
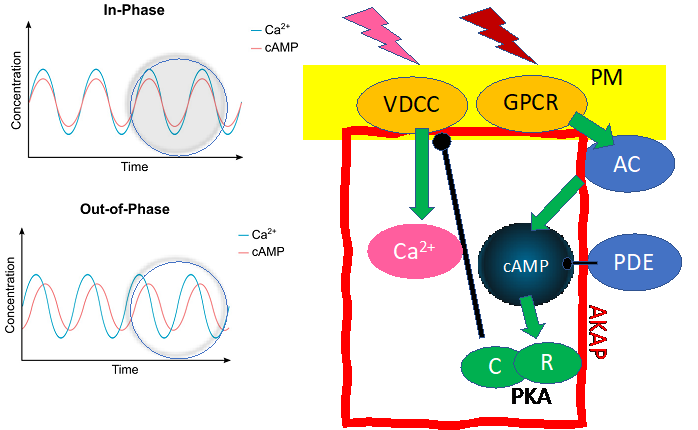
Figure 1: Plasma membrane (PM) master clock multiscale zeitgeber rhythms translated from extracellular stimuli via PM-receptors (GPCR, VDCC) are mirrored by multiscale oscillations of the second messengers Ca2+ and cAMP. These oscillations are coupled via second messenger-dependent molecules such as cyclases (AC), kinases (PKA R- and C-subunits), phosphodiesterases (PDE) and ion channels (VDCC). Ca2+ and cAMP signals are in-phase in the plasma membrane (PM) and out of phase inside the cell.
Approach and Methods
We hypothesize that modulating second messenger oscillations in distinct signaling compartments can affect temporal information processing in the entire cell. Thus, cAMP and Ca2+ concentrations are monitored in primary insect circadian clock neurons (Stengl) cultured on custom-made platforms (Popov) upon stimulation with zeitgeber signals or coupling factors while assessing the role of PKA as a master controller. We will examine whether intracellular signaling via cAMP and Ca2+ occurs at characteristically different time scales. With RNAi- dependent knockdown of AKAPs in primary cell cultures of cockroach circadian pacemaker neurons (Stengl) and mutant analysis in Drosophila embryos (Kapp/Müller) in vivo we will examine how multiscale timing requires compartmentalization of signaling cascades regulated via AKAPs. We can quantitatively describe the mode of action of second messengers such as cyclic nucleotides and derivatives thereof (fluorescent cAMP analogs, protein-isoform specific analogs, via direct measurement of protein-protein and ligand-protein interactions employing Surface Plasmon Resonance (SPR), switchSENSE, Isothermal Titration Calorimetry (ITC), Fluorescence polarization (FP), and Bioluminescence Resonance Energy Transfer (BRET, Mayer). Membrane permeable cyclic nucleotide analogues stapled peptides (FRET-based) sensors for Ca2+ and cAMP will be employed for simultaneous in vitro and in vivo real time imaging. With recombinant proteins and respective 3-dimensional structures of cAMP-targets available, we will combine biochemical, structural, and cell-based assays with data-based models and finite element simulations (Friedmann) to address collaborative projects for PhD-candidates.
future collaboration
new project of Prof. Kleinschmidt (Physics)
