Project 3
P3: Subcellular oscillations during syncytial cleavage divisions in Drosophila melanogaster
Project description
The embryogenesis of the fruit fly, Drosophila melanogaster, is an excellent model system in molecular cell biology. Embryogenesis in the fly starts with a zygote undergoing rapid mitotic divisions without intervening cytokineses. In this system, the cell cycle represents the master oscillator. It is coupled to rearrangements of the actin cytoskeleton and to membrane vesicle transport resulting in oscillating metaphase furrow formation. It is not well understood how the cell cycle oscillator is linked to these subcellular oscillations. Using live-imaging of fluorescently labelled markers to detect F-actin or membrane compartments in combination with fluorescently labelled tubulin as proxy for the cell cycle, the temporal-spatial parameters of individual subcellular events will be recorded in a quantitative manner. Moreover, the role of second messengers for F-actin rearrangements and membrane turnover during furrow formation will be addressed. These data will provide a basis for computer-based-modelling to further inform the experimental design for a mechanistic understanding of the process. The sophisticated genetic toolbox of the fly will be applied to characterize known candidate genes and to identify novel zygotic genes driving the coordination of subcellular oscillation with the cell cycle.
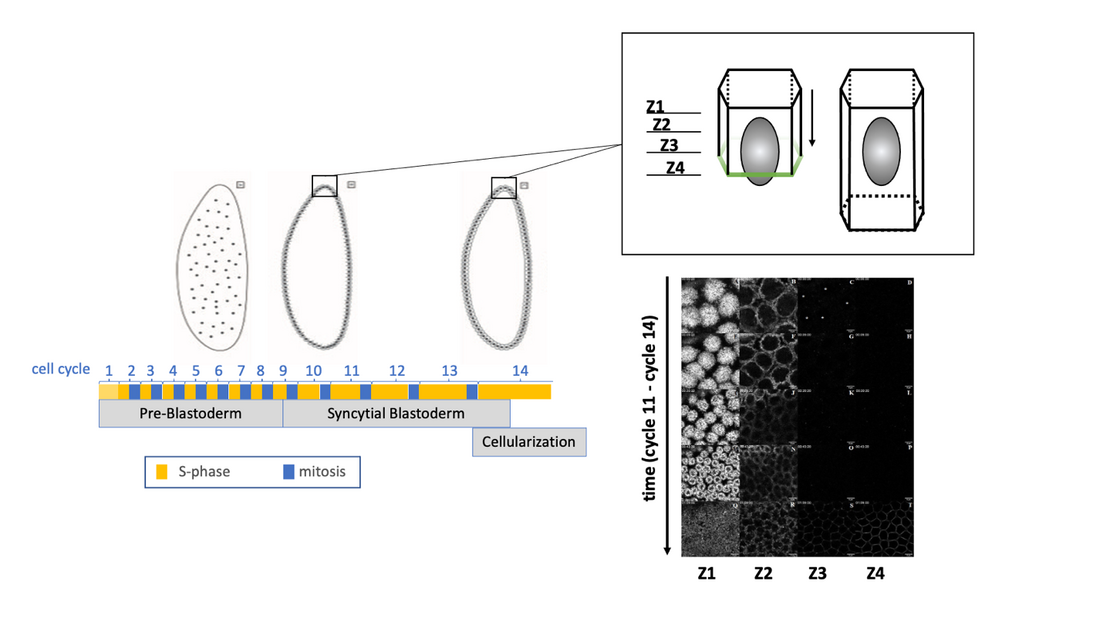
Figure 1: The cartoon on the left illustrates Drosophila early embryogenesis represented by 13 cell cycles, consisting of S-phases and mitoses but no intervening cytokineses, and resulting in a syncytial blastoderm. In interphase of cycle 14, cellularization takes place, during which a membrane furrow is inserted in between nuclei. Thereby, a single-layered epithelium is formed, as shown in the cartoon top right side. In this system, F-actin rearrangements can be recorded, as shown bottom right side, in a spatio-temporal manner.
